Labcourse and protocol are conducted and have to be written in English
- start: 9:00AM
- meeting point: S2|07 153
- location: S2|07 164
- experiment number: 3.35
- Instruction: Optics with 2D Quantum Materials (eng) (opens in new tab)
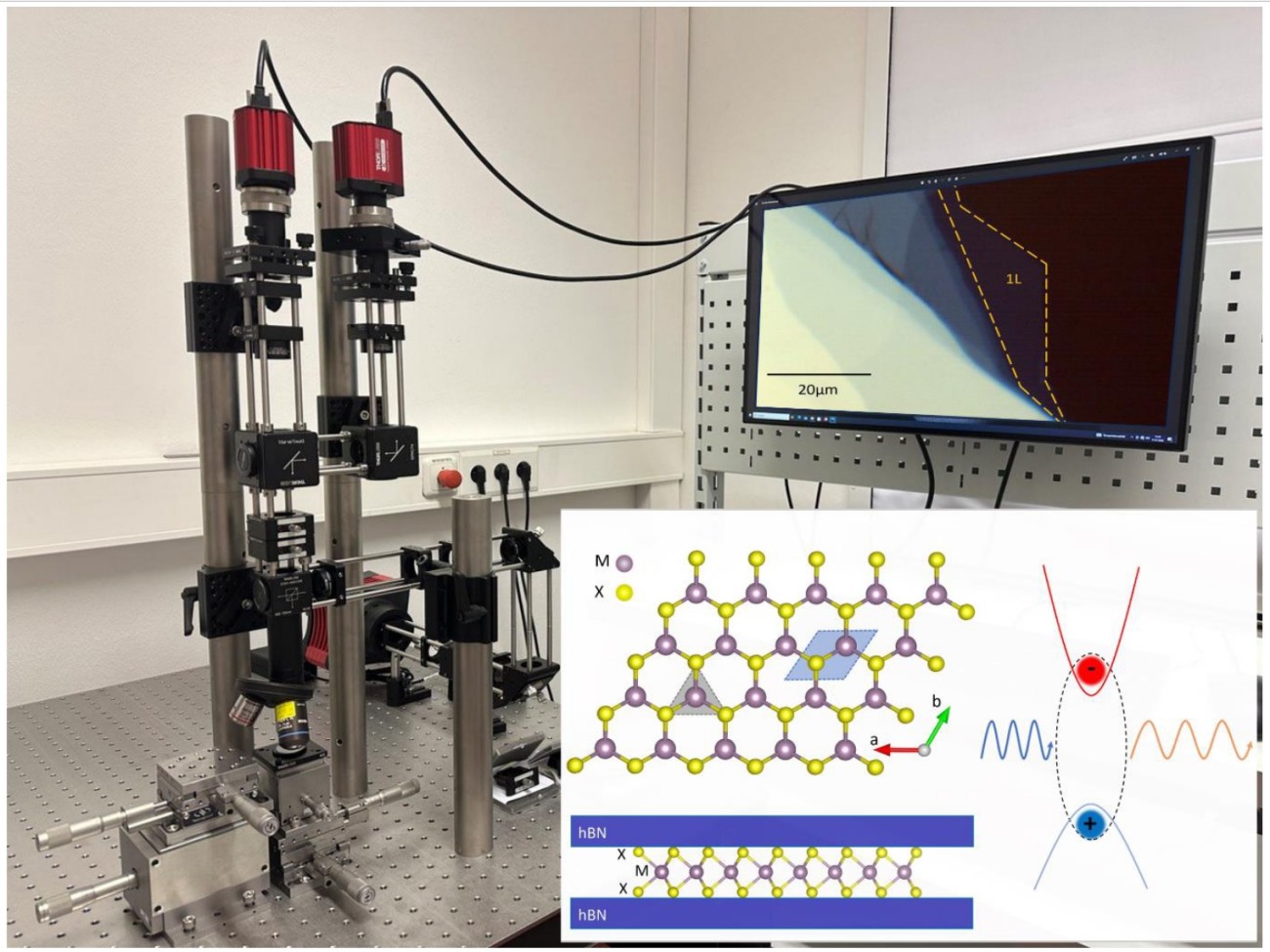
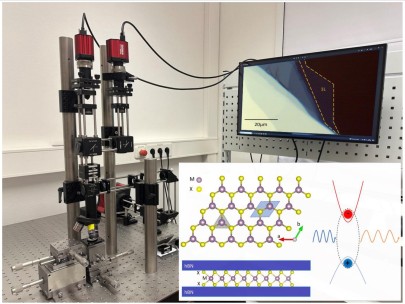
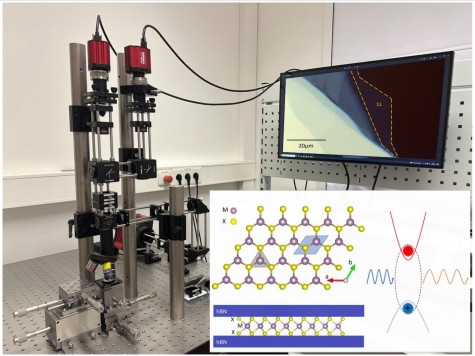
The successful isolation of Graphene, a single atomic layer of carbon, in 2004 started the era of 2D materials.[1] Here the strong bonds between atoms in the same layer are covalent, whereas the layers are held together by weak van der Waals forces to form a layered material. Properties of single or a few layers are governed by quantum mechanics and thus they exhibit distinctly different properties in comparison to large bulk crystals. 2D transition metal dichalcogenides (TMDs) crystals are particularly interesting for optics and electronics. Some examples of TMDs are the semiconductors WS2, WSe2 and MoS2 that emit light efficiently when thinned down to one monolayer. Layers of different 2D materials may also be combined to form so called van der Waals (vdW) heterostructures. By choosing which 2 materials we combine in a stacked bilayer and by deciding the stacking the angle between layers we can tune the properties of the new crystal, with properties ranging from superconductivity in multilayer graphene to tunable photoluminescence in TMD heterostructures have been observed.
The goals of this experiment are to:
- Introduce the optical properties of the 2D quantum materials.
- Develop an understanding of optical microscopy as a powerful tool for the analysis of atomic monolayers and their strong light-matter-interaction
- Image, measure and analyse the photoluminescence emission from atomically thin semiconductors and vdW heterostructures to understand the physical nature of different bandgaps and light emission mechanisms in quantum materials
Literatur
For this experiment, the literature is available both via the digital content of the ULB and via the publishers from within the university network (e.g. eduroam). The links are given in the References section of the experiment instructions.