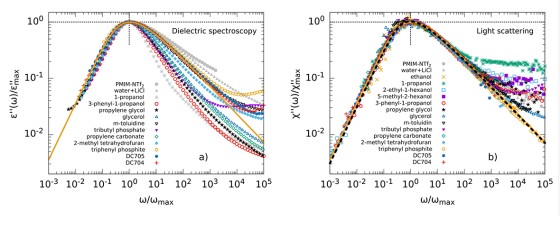
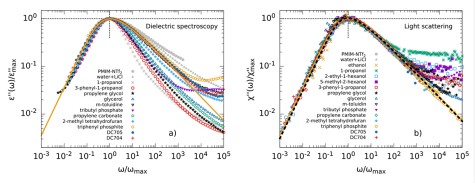
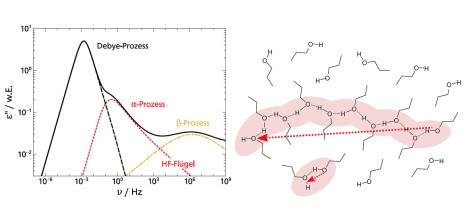
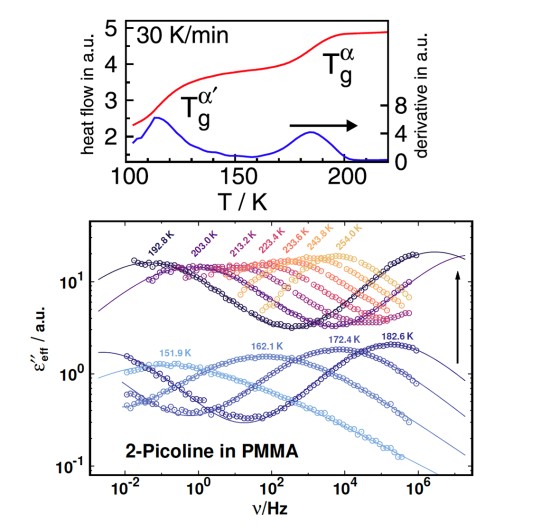
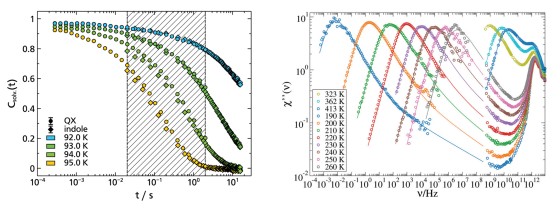
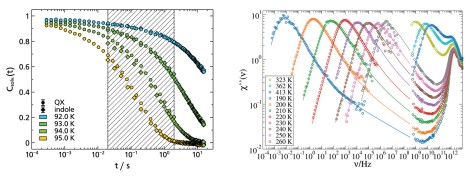
The dielectric loss ϵ′ in broadband dielectric spectroscopy (BDS) experiments as well as the imaginary part of the generalized susceptibility χ′ in depolarized dynamic light scattering (DDLS)experiments contains several peaks that can be assigned to different processes of molecular dynamics.Here, the spectral shape of the α-process is of particular interest, as it is linked to the macroscopic viscosity.
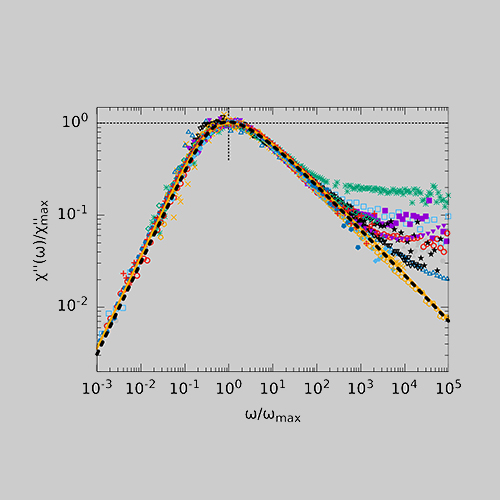
When comparing different glass-forming liquids in the BDS, the α-process shows strongly deviating shape parameters βKWW of the corresponding correlation function, which typically lie between 0.5 and 0.8. In DDLS, a different picture emerges. Here a large number of liquids show the same “generic” spectral shape which at high frequencies can be described by a power law ∝ ω−1/2. The figure shows data of various glass-forming liquids measured by BDS (left) and DDLS (right). The set of substances includes polar liquids with strongly different polarity, van der Waals liquids, hydrogen bonding systems and some ionic liquids.
In order to understand these discrepancies in spectral shape when using different methods, the methods must be considered in more detail. The experimentally determined correlation and response functions usually contain not only a self-correlation component but also contributions from cross-correlations which are due to interactions between molecules such as dipole-dipole interactions. BDS is particularly sensitive for the latter while DDLS provides more direct access to the self-correlation component. Thus, the deviation of the spectral shape from generic behavior in BDS could therefore possibly be explained by cross-correlations.