Because a crossover between two liquid phases of water should manifest itself in a change of the dynamical behavior, we study the temperature-dependent motion of water in nanopores by nuclear magnetic resonance and broadband dielectric spectroscopy. Our results show that confined water exhibits a dynamical crossover at ca. 220 K, which is largely independent of the pore size. Further studies are required to determine to what degree this finding can be taken as evidence for the existence of a liquid-liquid phase transition. In particular, it is necessary to analyze whether the dynamical crossover is related to the properties of bulk water or caused by the severe confinement.
Institute for Condensed Matter Physics

Water anomalies
Although water has a simple chemical structure, it shows numerous exceptional properties. A prominent example is the well-known density anomaly. Despite intensive research efforts, the origin of this and other anomalies of water is still under controversial scientific debate.
Are there two liquid phases of water?
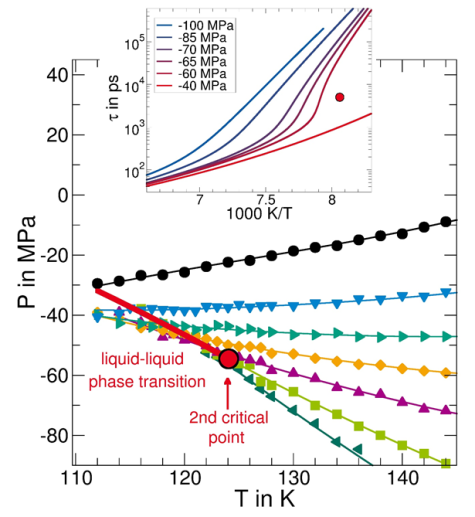
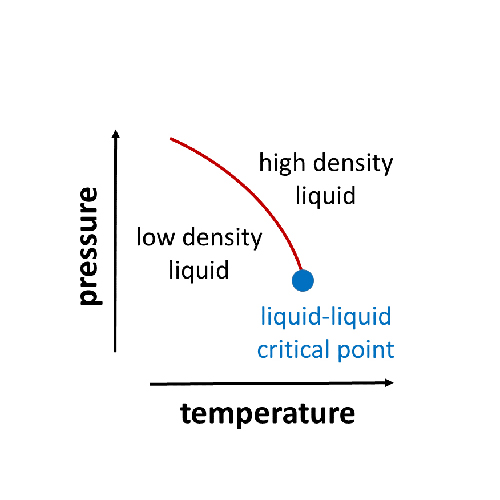
A much-noticed hypothesis postulates that the anomalies are caused by the existence of two liquid phases of water, which differ in density and are separated by a first-order phase transition at reduced temperatures and enhanced pressures. For tests of this hypothesis, it is a severe problem that the liquid phase cannot be stabilized for sufficiently long times in the relevant temperature and pressure ranges due to the high tendency of water for crystallization. Therefore, current research follows different routes to circumvent crystallization so as to ascertain the properties of deeply supercooled water.
Picture: AG Vogel
Experimental studies of deeply cooled water in confinement
It is well established that the crystallization of water is suppressed in narrow confinements with diameters of a few nanometers. Therefore, severely confined water provides access to the supercooled temperature range of the liquid.
Picture: AG Vogel
Simulations of deeply supercooled water in the bulk
In molecular dynamics simulations, a crystallization of water can be avoided by high cooling rates. Thus, suitable water models provide access to the properties of supercooled bulk water.
Selected publications
- Silvina Cerveny, Francesco Mallamace, Jan Swenson, Michael Vogel, and Limei Xu (2016). “Confined Water as Model of Supercooled Water”, Chemical Reviews 2016 116 (13), 7608-7625, https://doi.org/ 10.1021/acs.chemrev.5b00609
- M. Weigler, M. Brodrecht, G. Buntkowsky, and M. Vogel (2019). “Reorientation of Deeply Cooled Water in Mesoporous Silica: NMR Studies of the Pore-Size Dependence” The Journal of Physical Chemistry B 2019 123 (9), 2123-2134, https://doi.org/ 10.1021/acs.jpcb.8b12204
- R. Horstmann and M. Vogel (2021). “Relations between Thermodynamics, Structures, and Dynamics for Modified Water Models in Their Supercooled Regimes,” The Journal of Chemical Physics 154, no. 5 (February 7, 2021): 054502, https://doi.org/10.1063/5.0037080