For example, we perform NMR static field gradient experiments to measure the diffusion coefficients of the individual components as a function of concentration and temperature. The results show that there are strong couplings of polymer and ion motions. Thus, a high mobility of the polymer is crucial for a fast transport of the ions.
Institute for Condensed Matter Physics

Polymer composites and electrolytes
Materials on the basis of polymers, e.g., plastics, are of enormous importance in everyday life. In many applications, polymers are not found as pure materials but together with suitable additives. This offers the possibility to adjust the material properties in a wide range by a mixing of appropriate components.
Exact picture of polymer systems
For a systematic improvement of the material properties, it is important to control the interactions of the constituents. We use nuclear magnetic resonance (NMR) experiments and molecular dynamics (MD) simulations to characterize the structure and dynamics of materials on polymer basis to improve our understanding of the interplay of the components.
Picture: AG Vogel
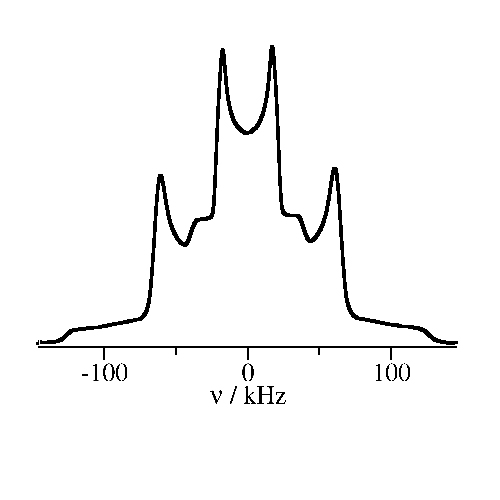
NMR experiments on polymer electrolytes
For a preparation of polymer electrolytes, it is exploited that a number of polymers can dissolve salts. The resulting mixtures feature fast ion transport, enabling a variety of applications in energy technologies. We use the isotope selectivity of NMR to investigate both polymer and ion dynamics in detail.
Picture: AG Vogel
Experiments and simulations on polymer nanocomposites
Addition of nanoparticles is often a very successful strategy to improve the material properties of polymers. For example, the mechanical and electrical properties of polymer electrolytes can usually be enhanced in this way. We perform experiments and simulations to study nanocomposite polymer electrolytes.
Selected publications
- Manuel Becher et al., “From Local to Diffusive Dynamics in Polymer Electrolytes: NMR Studies on Coupling of Polymer and Ion Dynamics across Length and Time Scales,” Macromolecules 52, no. 23 (December 10, 2019): 9128–39, https://doi.org/10.1021/acs.macromol.9b01400.
- André Bormuth et al., “Chain-Length Dependence of Polymer Dynamics: A Comparison of Results from Molecular Dynamics Simulations and Field-Cycling 1 H NMR,” Macromolecules 46, no. 19 (October 8, 2013): 7805–11, https://doi.org/10.1021/ma401198c.
- M. Weigler et al., “On the Microscopic Origins of Relaxation Processes in Aqueous Peptide Solutions Undergoing a Glass Transition,” The Journal of Chemical Physics 152, no. 23 (June 21, 2020): 234503, https://doi.org/10.1063/5.0010312.




