For example, we study water confined to porous silica with specifically modified inner surfaces. Our results show that the mobility of the water molecules strongly depends on the properties of a neighboring interface. In particular, we observe that the functionalization of the inner surfaces with amino acids can slow down the reorientation of water by up to 2 orders of magnitude. This effect is stronger for basic Lys than for acidic Glu, while neutral Ala plays an intermediate role. These results suggest that the biologically relevant water mobility at protein surfaces is controlled by the amino acid sequence.
Institute for Condensed Matter Physics

Liquids at interfaces
In nature and technology, liquids are often found in narrow confinements and, hence, near interfaces. For example, in geological and biological materials but also in modern applications, water is restricted to very narrow compartments. Hereby, many natural and technological confinements do not contain pure liquids, but mixed liquids. For instance, in biological environments, water and cosolvents reside between various types of biopolymers, which show spacings of only a few nanometers.
When small matters…
In general, the properties of a system can drastically change when its size is reduced to nanoscopic scales. Such reduction in size means that interface effects, which result from specific interactions at the boundaries, gain enormously in importance relative to volume effects. This leads to very complex and incompletely understood properties. Liquids in nano-confinements are, thus, of large interest from the viewpoints of both fundamental and applied sciences.
Several research projects on the topic “Hydrogen-bonded liquids subject to interfaces of various hydroaffinities” formed the DFG research unit FOR 1583.
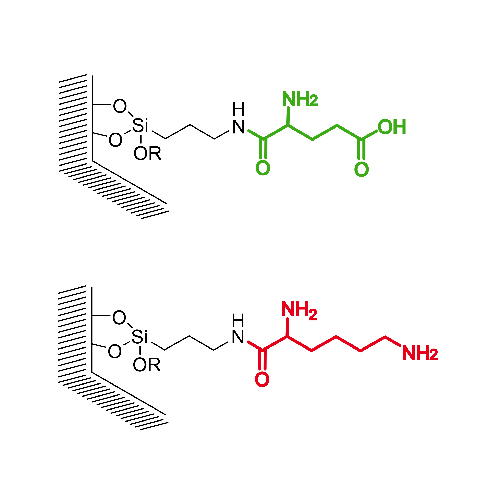
Picture: AG Vogel
Experiments on liquids at interfaces
The behavior of water in nanoscopic confinements is of particular relevance. It is well established that confinements with sizes of a few nanometers suppress the crystallization of water. We apply nuclear magnetic resonance and broadband dielectric spectroscopy to investigate water dynamics in these nanoscopic confinements.
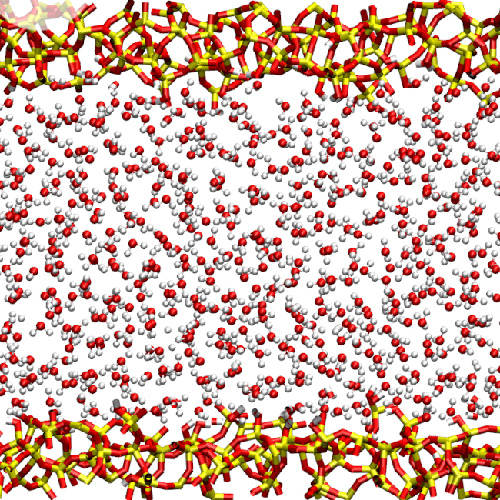
Picture: AG Vogel
Simulations of liquids at interfaces
Molecular dynamics simulations allow us to analyze the structure and dynamics of confined liquids in a very detailed and spatially resolved manner, e.g., as a function of the distance from an interface.
Selected publications
- Gerd Buntkowsky, Michael Vogel und Roland Winter (2018). “Properties of Hydrogen-Bonded Liquids at Interfaces” Zeitschrift für Physikalische Chemie, vol. 232, no. 7-8, 2018, pp. 937-972. https://doi.org/10.1515/zpch-2018-1110
- Julian Geske, Michael Harrach, Lotta Heckmann, Robin Horstmann, Felix Klameth, Niels Müller, Elvira Pafong, Timothy Wohlfromm, Barbara Drossel und Michael Vogel (2018). “Molecular Dynamics Simulations of Water, Silica, and Aqueous Mixtures in Bulk and Confinement” Zeitschrift für Physikalische Chemie, vol. 232, no. 7-8, 2018, pp. 1187-1225. https://doi.org/10.1515/zpch-2017-1042
- Gerd Buntkowski und Michael Vogel (2020). “Small Molecules, Non-Covalent Interactions, and Confinement” Molecules 25, no. 14: 3311. https://doi.org/10.3390/molecules25143311




