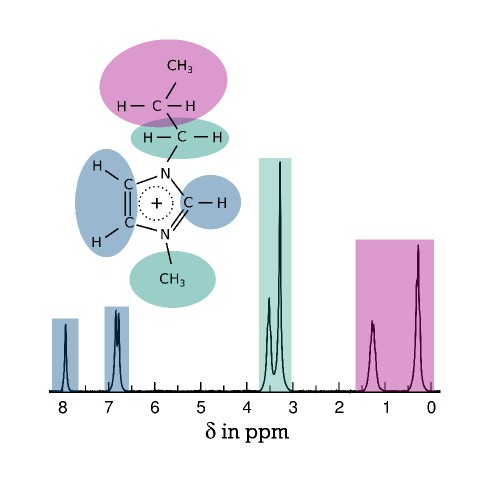
Our simulation results reveal that various ionic liquids with amphiphilic components feature extended polar and nonpolar regions, which grow upon cooling and form bicontinous phases at sufficiently low temperatures. Moreover, it is possible to distinguish ions with very different dynamics on intermediate time scales between ballistic and diffusive motions. Because studies of these spacious structural and dynamical heterogeneities require time-consuming simulations of very large systems, we collaborate with other research groups to develop computationally less expensive coarse-grained models of ionic liquids in the framework of a DFG funded collaborative research center (TRR 146).

Picture: AG Vogel

Picture: AG Vogel