Specifically, NMR studies allow us not only to obtain diffusion coefficients but also to measure correlation functions and spectral densities of the ionic motion in a straightforward manner. Moreover, temperature-dependent analysis provides access to the energy landscape of the ion transport. We exploit these capabilities to determine, e.g., the mechanisms for the charge transport in glass-ceramics, which exhibit promisingly high ion conductivities. Our results show that broad distributions of activation energies govern the ion jumps in these materials. Thus, the ions have largely different jump rates. This fact needs to be considered in theoretical models of the charge transport in battery materials.
Institute for Condensed Matter Physics

Ion transport in energy materials
The transport of ions in energy materials is of enormous technological interest. Prominent examples are the motion of lithium ions in solid materials for battery applications or the migration of protons in polymer membranes for fuel cells. Most technological applications require fast ion transport. Therefore, the ions need to show high mobility in the solid or polymer matrix.
Understanding ion motions
Our goal is a comprehensive characterization of the ion dynamics so as to improve our understanding of this motion and to identify factors that limit the rate of the charge transport and, thus, of the technological applications. For this purpose, we employ nuclear magnetic resonance (NMR). In particular, we exploit that NMR provides access to both short-range and long-range ion dynamics so that microscopic and macroscopic properties can be correlated.
Picture: AG Vogel
NMR experiments on ion transport in solid electrolytes
In solid electrolytes, the charge transport results from jumps of mobile ions between defined sites in a rigid matrix. NMR experiments enable a detailed characterization of this jump motion.

Picture: AG Vogel
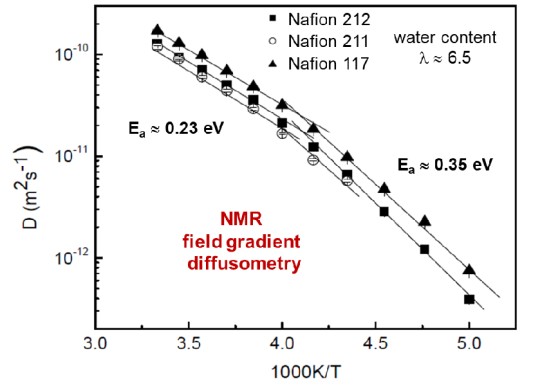
NMR experiments on proton transport in polymer membranes
Fuel cells based on polymer-exchange membranes are promising to become the next generation of energy conversion devices.
Selected publications
- Roland Böhmer, Kenneth.R. Jeffrey and Michael Vogel (2006). “Solid-state Li NMR with applications to the translational dynamics in ion conductors” Progress in Nuclear Magnetic Resonance Spectroscopy, Volume 50, Issues 2–3, Pages 87-174, ISSN 0079-6565, https://doi.org/10.1016/j.pnmrs.2006.12.001.
- Michael Haaks, Steve W. Martin and Michael Vogel (2017), “Relation of short-range and long-range lithium ion dynamics in glass-ceramics: Insights from 7Li NMR field-cycling and field-gradient studies” Phys. Rev. B 96, 104301, DOI: 10.1103/PhysRevB.96.104301
- Edda Winter, Philipp Seipel, Vanessa Miß, Stefan Spannenberger, Bernhard Roling, and Michael Vogel (2020). “7Li NMR Studies of Short-Range and Long-Range Lithium Ion Dynamics in a Heat-Treated Lithium Iodide-Doped Lithium Thiophosphate Glass Featuring High Ion Conductivity” The Journal of Physical Chemistry C 2020 124 (52), 28614-28622, DOI: 10.1021/acs.jpcc.0c08801